What is mole percent?
1 Answer
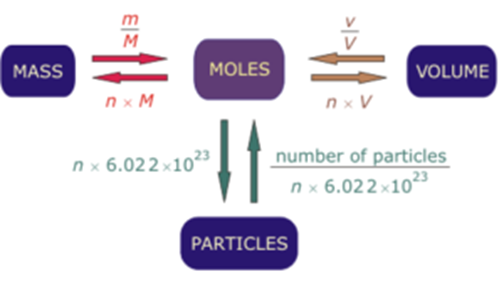
A mole is like a dozen. It is a name for a specific number of things. There are 12 things in a dozen, and 602 hexillion things in a mole. We'll talk about wh. As you already know how the grams to moles conversion work, find the number of moles: n = 5988 g / 18.015 g/mol = 332.4 mol. You can always use our grams to moles calculator to check the result! Knowing how to convert grams to moles may be helpful in numerous chemical tasks, e.g., finding the mole fraction of a solution. I like fill-in-the-blank days. However, there is a problem with mole day. Mole day is, of course, 10/23. You know, a mole? One mole is 6.02 x 1023 Avogadro’s number.
Warning! Long Answer. Mole percent is the percentage that the moles of a particular component are of the total moles that are in a mixture.
Explanation:
MOLE FRACTION
Let’s start with the definition of mole fraction.
Mole fraction
The mole fraction of component a is
Mole Number Relationships
If a system consists of only two components, one component is the solvent and the other is the solute.
The more abundant component, the solvent, is usually called component 1, and the solute is called component 2.
/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)
The sum of the mole fractions for each component in a solution is equal to 1. For a solution containing two components, solute and solvent,
MOLE PERCENT
Mole percent is equal to the mole fraction for the component multiplied by 100 %
mole % a =
The sum of the mole percentages for each component in a solution is equal to 100 %.
For a solution containing two components, solute and solvent,
mole % solute + mole % solvent = 100 %
EXAMPLE
Avogadro's Number
What are the mole fraction and mole percent of sodium chloride and the mole fraction and mole percent of water in an aqueous solution containing 25.0 g water and 5.0 g sodium chloride?
(a) Identify the components making up the solution.
(b) Calculate the moles of each component.
(c) Calculate the mole fraction of each component.

[Note: We could also have written
(d) Calculate the mole percent of each component.
Mole % 2 =
Mole % 1 =
[Note: We could also have written mole % 1 = 100 – mole % 2 = (100 – 5.8) % = 94.2 mole %]
Summary
Related questions
