- Relative Atomic Mass Of Sodium Chloride
- Atomic Mass Calculation Formula
- Relative Atomic Mass Of Sodium Chloride
How do you calculate relative formula mass?
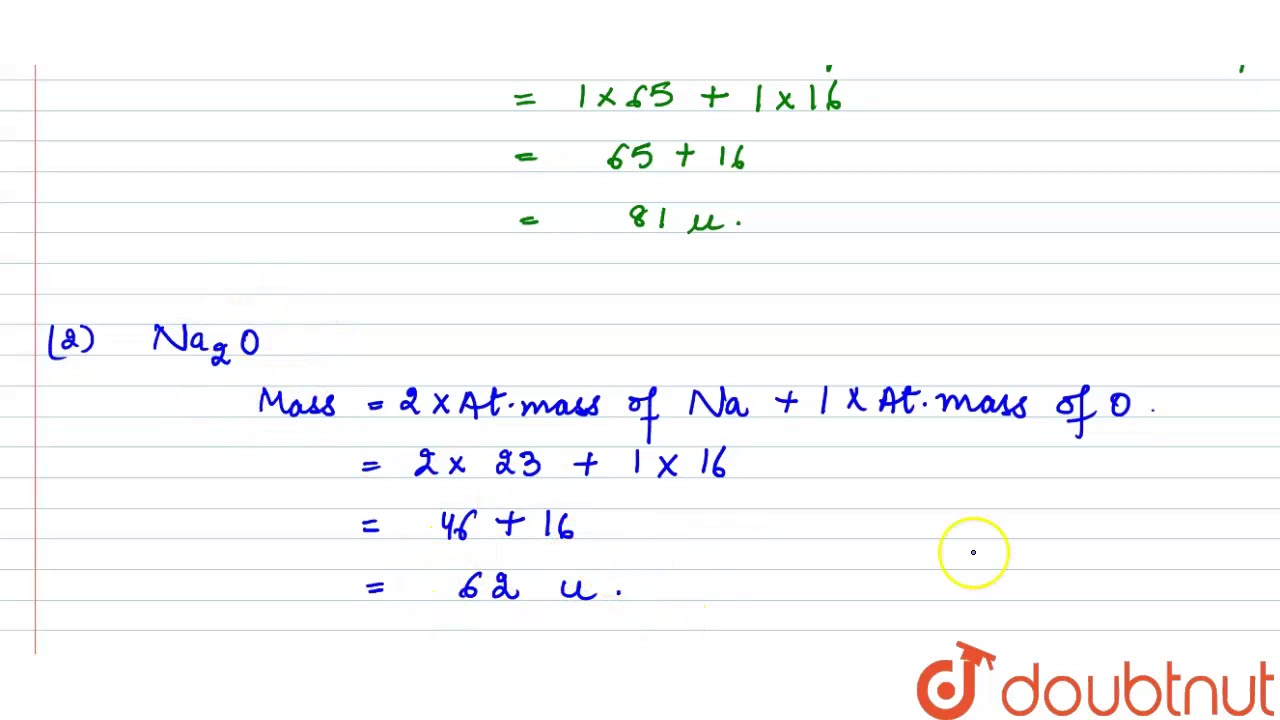
Relative Atomic Mass Of Sodium Chloride
However, since these are relative masses of the atoms any mass unit can be used, as shown by the two columns to the right of the arrows, above. In terms of atomic masses: in a total of 142.02 parts by mass of sodium sulfate, 45.98 parts are sodium, 32.06 parts are sulfur, and 64.00 parts are oxygen.
1 Answer
- Generally, Avogadro number of atoms of sodium weigh 23 g. = 6.022. 10^23 atoms weigh 23 grams = 1 atom weighs 23/(Avogadro number) grams = 3.8. 10^-23 grams.
- Isotope abundances of sodium. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This is not to be confused with the relative percentage isotope abundances which totals 100% for all the naturally occurring isotopes.
- Generally, Avogadro number of atoms of sodium weigh 23 g. = 6.022. 10^23 atoms weigh 23 grams = 1 atom weighs 23/(Avogadro number) grams = 3.8. 10^-23 grams. So, one atom of sodium weighs 3.8. 10^-23 grams (approximately).
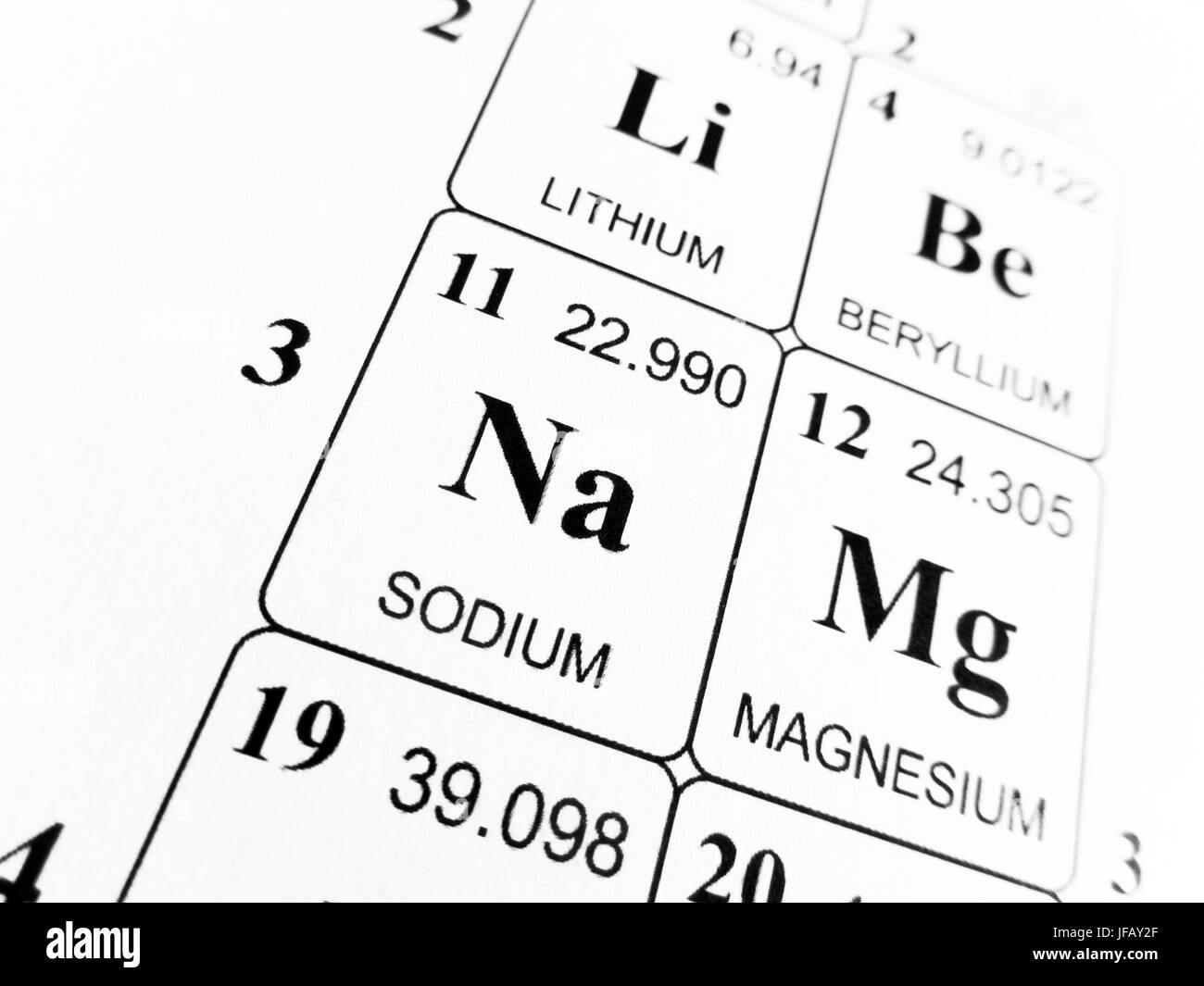
- When you look at a periodic table of the elements, you see that sodium has an atomic weight of 23. This means that a single atom of sodium weighs 23 atomic mass units (AMUs), but it also means that.
Explanation:
Relative formula mass,

Example: What is the relative formula mass for sodium sulfate,
Atomic Mass Calculation Formula
When working with moles, the relative atomic and formula masses are numerically equal the molar mass. Molar mass is the mass in grams of one mole of a substance. So the mass of one mole of sodium,
Relative Atomic Mass Of Sodium Chloride
Related questions
