- Atomic Number Is Equal To Number Of Neutrons
- Atomic Number Is Equal To Protons And Electrons
- What Makes Up The Atomic Number
Atomic Number vs Mass Number
Atoms are characterized by their atomic numbers and mass numbers. In the periodic table, atoms are arranged according to their atomic number. Mass number of an element is more related with the mass of it. However, it is not giving the exact mass of the atom. There are some elements, where atomic number and mass number is similar, and most of the time, mass number is higher than the atomic number.
Its chemical behavior is determined by the atomic number 2 (the number of protons), which equals the normal number of electrons; the stability of its nucleus (that is, its radioactivity) varies with its atomic weight (approximately equal to the number of protons and neutrons). The most well-known form of plutonium, for example, has an atomic. Atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
What is Atomic Number?
- So the atomic number is symbolized by Z and it refers to the number of protons in a nucleus and you can find the atomic number on the periodic table so we're going to talk about hydrogen in this video so for hydrogen hydrogen's atomic number is 1 so it's right here so there's one proton in the nucleus of a hydrogen atom in a neutral atom the number of protons is equal to the number of.
- A neutral atom's atomic number is equal to. A) the number of nucleons B) the sum of protons and neutrons. C) the number of electrons D) the number of neutrons 2) Calcium carbonate (CaCO 3) is 34.5% Ca by mass. In a 418 g sample of CaCO 3, how many grams of calcium are present?
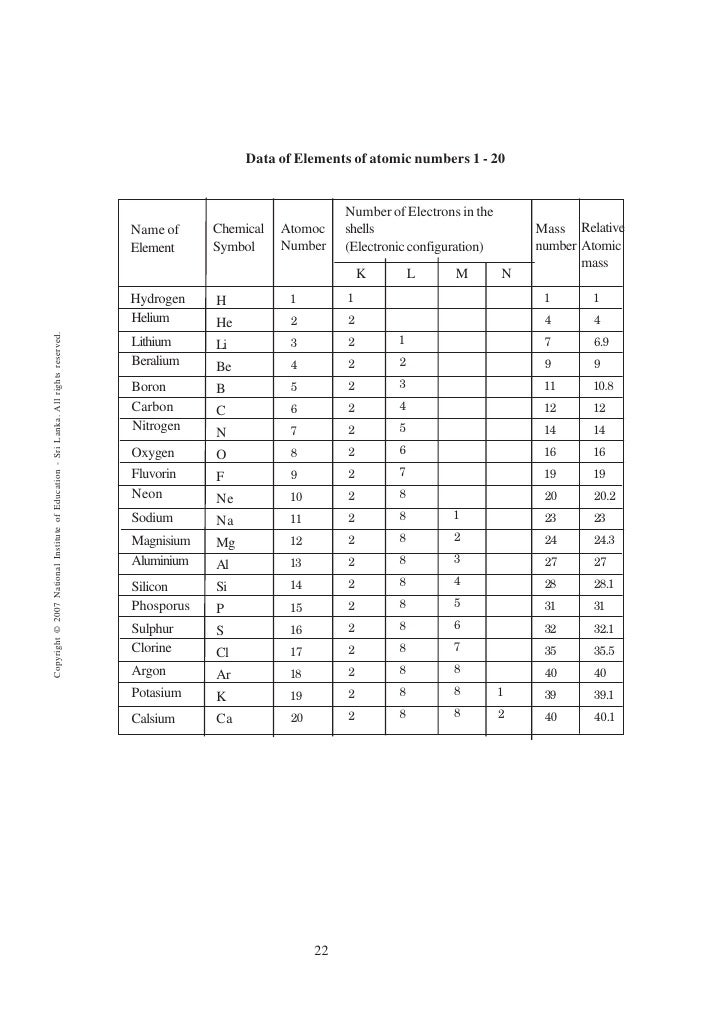
Atomic number of an element is the number of protons it has in the nucleus. The symbol for denoting the atomic number is Z. When the atom is neutral, it has the same number of electrons as protons. Thus, atomic number is equal to the number of electrons in this instance. But it is always reliable to get the number of protons as the atomic number. The elements in the periodic table are arranged according to the increasing atomic number. This arrangement has automatically arranged them in increased atomic weight most of the time. Every element has separate atomic number, and no element has the same atomic number. Therefore, atomic number is a convenient way of distinguishing different elements. By looking at the atomic number itself, a lot of information about the element can be withdrawn. For example, it tells the group and the period where the element belongs in the periodic table. Further, it gives information about oxidation states, charge of the ion, bonding behavior, nucleus charge, etc.

What is Mass Number?
Atoms are mainly composed of protons, neutrons and electrons. Atomic mass is simply the mass of an atom. Most of the atoms in the periodic table have two or more isotopes. Isotopes differ from each other by having a different number of neutrons, even though they have the same proton and electron amount. Since their neutron amount is different, each isotope has a different atomic mass.
Mass number is the total number of neutrons and protons in a nucleus of an atom. The collection of neutrons and protons is also known as nucleons. Therefore, mass number can also be defined as the number of nucleons in a nucleus of an atom. Normally, this is denoted in the left, upper corner of the element (as superscript) as an integer value. Different isotopes have different mass numbers, because their numbers of neutrons vary. Therefore, the mass number of an element gives the mass of the element in integers. The difference between the mass number and the atomic number of an element gives the number of neutrons it has.
What is the difference between atomic number and mass number? • Atomic number is the number of protons in a nucleus of an atom. Mass number is the total number of protons and neutrons in the nucleus. • Conventionally atomic number is written in the left, bottom corner of an element, whereas the mass number is written in the left, upper corner. • Atomic number is denoted by Z, and mass number is denoted by the symbol A. |
Related posts:
| Fundamental Subatomic Particles | Electromagnetic Radiation |
| Light and Other Forms of Electromagnetic Radiation | |
| Particle | Symbol | Charge | Mass | |
| electron | e- | -1 | 0.0005486 amu | |
| proton | p+ | +1 | 1.007276 amu | |
| neutron | no | 0 | 1.008665 amu | |
The number of protons, neutrons, and electrons in an atom can be determined from a set of simple rules.
- The number of protons in the nucleus of the atom is equal to the atomic number (Z).
- The number of electrons in a neutral atom is equal to the number of protons.
- The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.
- The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
Examples: Let's determine the number of protons, neutrons, and electrons in the following isotopes.
The different isotopes of an element are identified by writing the mass number of the atom in the upper left corner of the symbol for the element. 12C, 13C, and 14C are isotopes of carbon (Z = 6) and therefore contain six protons. If the atoms are neutral, they also must contain six electrons. The only difference between these isotopes is the number of neutrons in the nucleus.
12C: 6 electrons, 6 protons, and 6 neutrons
13C: 6 electrons, 6 protons, and 7 neutrons
14C: 6 electrons, 6 protons, and 8 neutrons
| Practice Problem 1: Calculate the number of electrons in the Cl- and Fe3+ ions. |
Much of what is known about the structure of the electrons in an atom has been obtained by studying the interaction between matter and different forms of electromagnetic radiation. Electromagnetic radiation has some of the properties of both a particle and a wave.
Particles have a definite mass and they occupy space. Waves have no mass and yet they carry energy as they travel through space. In addition to their ability to carry energy, waves have four other characteristic properties: speed, frequency, wavelength, and amplitude. The frequency (v) is the number of waves (or cycles) per unit of time. The frequency of a wave is reported in units of cycles per second (s-1) or hertz (Hz).
The idealized drawing of a wave in the figure below illustrates the definitions of amplitude and wavelength. The wavelength (l) is the smallest distance between repeating points on the wave. The amplitude of the wave is the distance between the highest (or lowest) point on the wave and the center of gravity of the wave.
If we measure the frequency (v) of a wave in cycles per second and the wavelength (l) in meters, the product of these two numbers has the units of meters per second. The product of the frequency (v) times the wavelength (l) of a wave is therefore the speed (s) at which the wave travels through space.
vl = s
| Practice Problem 2: What is the speed of a wave that has a wavelength of 1 meter and a frequency of 60 cycles per second? |
| Practice Problem 3: Orchestras in the United States tune their instruments to an 'A' that has a frequency of 440 cycles per second, or 440 Hz. If the speed of sound is 1116 feet per second, what is the wavelength of this note? |
Light is a wave with both electric and magnetic components. It is therefore a form of electromagnetic radiation.
Visible light contains the narrow band of frequencies and wavelengths in the portion of the electro-magnetic spectrum that our eyes can detect. It includes radiation with wavelengths between about 400 nm (violet) and 700 nm (red). Because it is a wave, light is bent when it enters a glass prism. When white light is focused on a prism, the light rays of different wavelengths are bent by differing amounts and the light is transformed into a spectrum of colors. Starting from the side of the spectrum where the light is bent by the smallest angle, the colors are red, orange, yellow, green, blue, and violet.

As we can see from the following diagram, the energy carried by light increases as we go from red to blue across the visible spectrum.
Because the wavelength of electromagnetic radiation can be as long as 40 m or as short as 10-5 nm, the visible spectrum is only a small portion of the total range of electromagnetic radiation.
The electromagnetic spectrum includes radio and TV waves, microwaves, infrared, visible light, ultraviolet, x-rays, g-rays, and cosmic rays, as shown in the figure above. These different forms of radiation all travel at the speed of light (c). They differ, however, in their frequencies and wavelengths. The product of the frequency times the wavelength of electromagnetic radiation is always equal to the speed of light.
vl = c
Atomic Number Is Equal To Number Of Neutrons
As a result, electromagnetic radiation that has a long wavelength has a low frequency, and radiation with a high frequency has a short wavelength.
Atomic Number Is Equal To Protons And Electrons
| Practice Problem 4: Calculate the frequency of red light that has a wavelength of 700.0 nm if the speed of light is 2.998 x 108 m/s. |
What Makes Up The Atomic Number
